Spinal Cord Stimulator Malpractice
Negligent Spinal Cord Stimulator Implant Lawsuit
After suffering from chronic back or neck pain that remains even conservative treatments or surgery, many patients choose to proceed with the insertion of a Spinal Cord Stimulator (SCS) to help manage their pain. Spinal cord stimulators can provide pain relief for thousands of patients each year, but the placement of the devices comes with risks if a surgeon or surgical team does not follow proper procedures.
For some pain victims, the placement of a spinal cord stimulator can cause irreversible harm including paralysis, leaving victims wondering where to turn next. If you were paralyzed or suffered a spinal cord injury during the placement of a spinal cord stimulator and want to learn more about your rights to recovery for your personal injuries or need answers to whether your complications were caused by medical malpractice, do not hesitate to seek legal assistance.
**Spinal Cord Stimulator Malpractice Lawsuits are not for the failure of a specific product, but rather Medical Negligence related to the device being implanted**
The most common medical negligence or medical malpractice cases arising from spinal cord stimulators occur due to inadequate pre-operative planning, improper surgical technique and monitoring and failing to remove the leads quickly if and when complications such as the inability to move arms or legs or bowel/bladder issues are noted in the recovery room.
The longer the leads remain in place if complications from spinal cord compression are occurring, the more likely permanent spinal cord injury will occur. Surgeons and recovery room nurses have a duty to recognize and respond to complications following the placement of a spinal cord stimulator. Failure to do so can constitute medical malpractice.
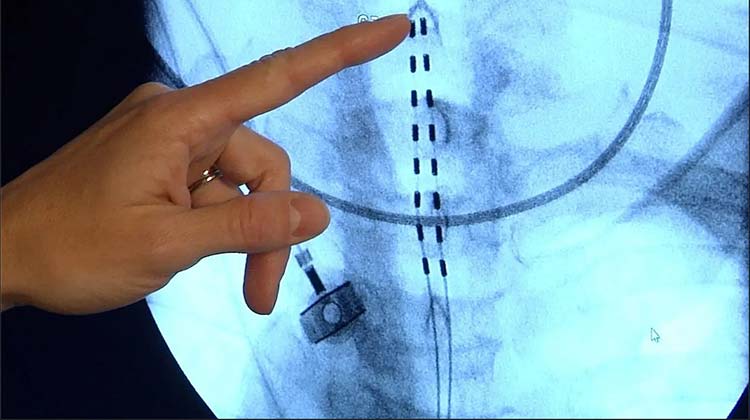
The experienced spinal cord stimulator malpractice attorneys at Miller Weisbrod Olesky are available to evaluate your potential case and provide a legal avenue if your injuries and complications were preventable. We have represented victims of medical malpractice and their families across the United States and won over 300 cases involving multi-million dollar recoveries. We are here to help you and your family seek and receive full justice.
Orthopedic and Pain Management Negligence case involving the improper implantation of a cervical spine stimulator resulting in our client becoming a quadriplegic. The Medical Malpractice Lawyers of Miller Weisbrod Olseky obtained $2,400,000 for our client!.
Our history in representing medical malpractice victims suffering from paralysis and other spinal cord injuries after the placement of spinal cord stimulators can be traced back to the late 1990’s. In an early case, we represented a victim and sued the orthopedic surgeon, pain management physician and the device manufacturer for negligence that resulted in the paralysis of our client. Following a jury verdict the manufacturer changed its labeling information on its spinal cord stimulators to warn that pre-operative imaging should be performed to confirm that there was adequate room for the stimulator leads to be placed without injuring the spinal cord.
Unfortunately, some doctors do not follow these warnings and some manufacturers still do not have clear warnings about the pre-surgery imaging requirements. Since our first case, we have represented numerous other victims of spinal cord stimulator complications.
What Is a Spinal Cord Stimulator Implant?
A spinal cord stimulator is a $30,000 implantable, paddle-shaped medical device used as a type of therapy to help relieve chronic pain when traditional surgical methods have failed. The implant is made up of thin wire electrodes attached to a battery-powered generator. A surgeon implants these electrodes in the spaces around the spinal cord and the vertebrae.
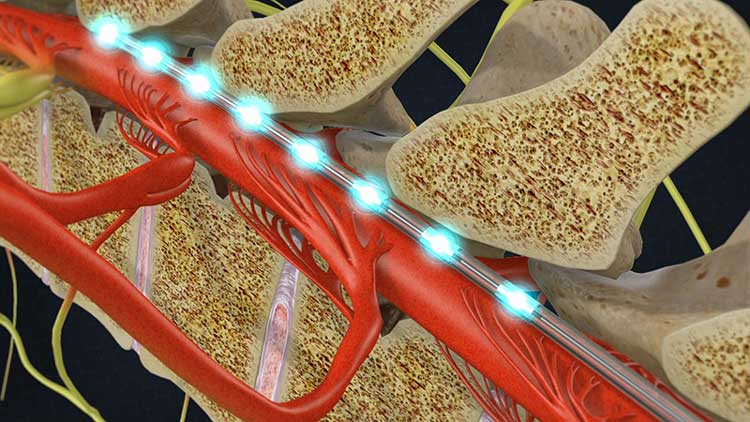
The surgeon then places the generator under the skin near the abdomen, buttocks, or in the patient's hip. The therapy treats chronic pain by emitting electrical impulses that produce a tingling sensation. This slight tingling sensation alters the patient's perception of pain by interrupting pain signals before they reach the brain. The patient can control the stimulator using a remote-control device. In some cases, a spinal cord stimulator for chronic pain can offer massive relief and allow patients suffering from chronic pain to lead a productive and more normal life.
For a patient to be considered as a candidate for this device, their physician must show that conservative treatments were used and failed to help. Additionally, the patient will undergo a psychological assessment to evaluate the likelihood of success. Once the patient has completed these psychological assessments, they will go through a three to seven-day trial period. During this trial period, the patient will have thin electrodes inserted under their skin. If the patient experiences relief from the external transmitter sending electrical pulses, they will undergo surgery to have the permanent stimulator implanted.
Medical Malpractice case involving the incorrect placement of a spinal cord stimulator resulting in our client suffering a severe brain injury. The Medical Malpractice Attorneys of Miller Weisbrod Olseky obtained a settlement of $2,200,000 for our client!.
How Spinal Cord Stimulators are Placed for Pain Relief?
Spinal cord stimulator systems are made up of two parts: a battery that powers the device and leads for delivering the stimulation. The two categories of leads that spinal cord stimulator systems utilize are percutaneous and paddle.
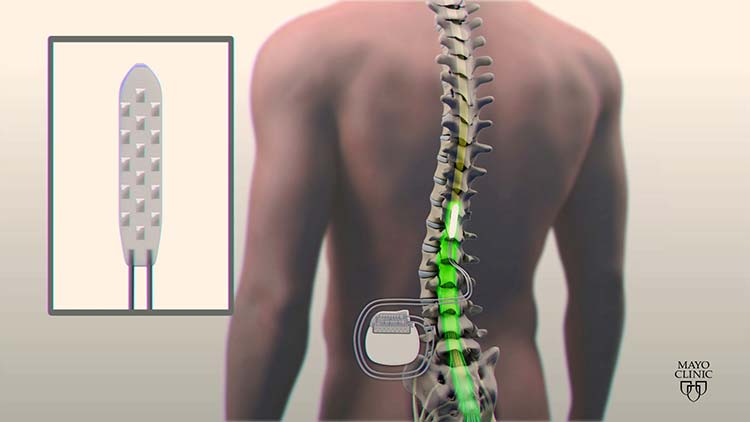
With an epidural needle, percutaneous leads are inserted into the spine. Percutaneous leads are are smaller leads. Paddle leads are larger than percutaneous leads. Paddle lead procedures typically requires an incision being made over your spine.
To place a paddle electrode, your doctor will need to create a space through muscle tissue for the electrode to fit. By contrast, when placing a percutaneous electrode, a doctor won't have to create that kind of space.
What are the Limitations of a Spinal Cord Stimulator?
Medical device companies and doctors have endorsed spinal cord stimulators as an effective solution for millions of patients suffering from a wide range of pain disorders. It's even been suggested that these devices can be used as a safe antidote to resolve the opioid crisis in the U.S.
However, an Associated Press News (AP) investigation found that these devices are actually more dangerous than many patients may realize. In fact, spinal cord stimulator implants account for the third-highest number of medical device injury reports to the Food and Drug Administration (FDA) from 2008 to 2017.
Furthermore, since 2018, the FDA has received more than 80,000 adverse event reports caused by SCS devices, with about 500 of these reports resulting in fatal outcomes. Other reports involved patients being shocked, burned, and suffering from spinal cord stimulator infections or spinal cord nerve damage ranging from muscle weakness to paraplegia.
Medical Malpractice lawsuit involving an attempted placement of a spinal cord stimulator leading to our client becoming paralyzed. The Medical Malpractice Attorneys of Miller Weisbrod Olseky obtained a settlement of $2,100,000 for our client!.
Some of the other significant risks that SCS devices include:
- The stimulator can move or become damaged.
- The stimulation may stop working or only work intermittently.
- Over-stimulation may occur and cause discomfort.
How Can an Implanted Spinal Cord Stimulator Cause Injury?
Spinal Cord Stimulators (SCS) can benefit those who suffer from chronic and severe pain in several ways. However, implanting the devices can cause a serious spinal cord injury if the surgeon and surgical team does not follow proper medical protocols known as the medial “standard of care”.
Here are some examples of how medical negligence in a spinal cord stimulator procedure can result in a spinal cord injury.
- The surgeon may improperly place leads that are too large to fit in a compromised or narrow spinal canal causing spinal cord injury.
- The surgeon may alter the leads making improper placement more likely during the operation.
- The surgeon may not have the patient awake during the procedure so that the can give feedback if the lead hits the spinal cord during placement and/or fail to have neuromonitoring (with evoked potentials) performed during surgery to notify the team of any spinal cord injury.
- The surgeon or neuromonitoring technician and remote monitoring neurologist may not correctly interpret or utilize the neuromonitoring technology to avoid spinal cord injury.
- The results of a spinal cord stimulator trial were not properly interpreted.
- The doctor fails to evaluate a candidate properly for surgery to implant a spinal cord stimulator.
- A manufacturing representative in the operating room may fail to warn the surgeon of an improper placement or surgical technique.
- The neuromonitoring tech and remote monitoring neurologist may fail to warn the surgeon when the neuromonitoring data shows spinal cord impingement which requires immediate removal of the leads.
- The placement of the leads causes bleeding leading to a hematoma (blood clot) that is not timely recognized and removed causing the spinal cord to be permanently injured due to the hematoma’s compression on the cord
Spinal Cord Stimulator Implant:
Failing to Select a Safe Insertion Site
A critical part of preparing for and performing a spinal cord stimulator placement involves carefully review MRI films to fully understand anatomy of the spine and spaces around the spine where the leads must be properly placed to provide safe and effective pain relief. A spinal cord should never be placed without a recent pre-operative MRI of the area of the spine or neck where the leads are to be placed.
A surgeon who performs spinal cord stimulator implant surgery must be aware of the soft bony tissue or bony growth (often caused by arthritis caused degenerative disc disease) found in the epidural space of the spinal canal. The surgeon must also be aware of the diameter of the spinal cord canal which can be reduced by stenosis or a narrowing by bone or a herniated or bulging disc.
The reason to understand these can conditions is because they can cause inadequate or limited epidural space for placement of the lead. This can make it difficult for a paddle lead to be inserted in the appropriate area.
If a surgeon operates on someone's spine without obtaining pre-insertion imaging of the spine or necessary information to select a safe insertion site, they may be held responsible for improperly inserting a spinal cord stimulator device that caused a permanent injury to the spinal cord. If a surgeon misinterprets films or fails to take unusual anatomic changes into account during the spinal cord stimulator surgery, this may fall below the standard of care known as medical negligence or medical malpractice.
Our client suffered additional Spinal Cord Damage during the implantation of a Spinal Cord Stimulator. Our Medical Malpractice Attorneys obtained a settlement of $1,873,000 for our client!.
Spinal Cord Stimulator Implant:
Failing to Properly Monitor a Patient's Course of Recovery
During a patient's recovery room time after spinal cord stimulator placement, a surgeon and the recovery room nurses and medical team must closely monitor the patient and quickly respond if they experience disproportionate pain, numbness, or weakness in their upper or lower extremities, or are unable to bear weight on their legs or lose grip or arm strength.
If complications are observed in the recovery room, the spinal cord stimulator patient must be quickly returned to surgery. When the spinal cord stimulator patient is returned to surgery, the surgeon should remove the stimulator leads and decompress the spinal cord to restore neurological function. Any delay in “decompressing” the spinal cord can result in a permanent spinal cord injury and constitutes medical negligence/medical malpractice.
If the surgeon or recovery room nurses and medical team fails to properly monitor the patient's recovery and fail to recognize or respond in a timely manner to any complications such as loss of function, this may constitute a breach of the standard of care (medical negligence/malpractice) for any resulting injuries, such as paralysis or bowel and bladder incontinence that could have been avoided or lessened with appropriate post-surgical care.
PRE and POST-OP Spinal Cord Stimulator Surgical Errors
When a person is scheduled for spinal cord stimulator placement surgery, they often worry about whether everything will go smoothly during the procedure. However, what the patient is often unaware of is that pre- and post-operative care or lack of care can cause just as much harm as an error made during surgery. Medical mistakes made before and after surgery may involve healthcare professionals, hospital support staff, or insufficient policies and procedures.
The following are some of the most common pre- and post-operative errors:
Failing to Properly Evaluate a Patient Before Surgery
Every surgery carries risks, and what those risks may be in a particular surgery will often depend on a thorough evaluation of a patient's medical history and current condition. Before the spinal cord stimulator procedure, a surgeon and the nursing team should give the patient a pre-operative blood test and assess their heart, liver, and lung function. Failing to ensure a patient is well enough to undergo surgery can have deadly consequences.

Incorrect or Inadequate Pre-Operative Information
When it comes to a spinal cord stimulator surgical procedure, even the most basic information should be verified multiple times. Although rare, a surgeon may receive inaccurate information from other medical personnel and operate in the wrong area. On the day of your spinal cord stimulator surgery, several people on your operative team must confirm your identity, the reason you are having surgery, and on what part of your body.
Failing to Closely Monitor the Patient After Surgery
Surgeons, nurses and other post-recovery medical providers must carefully monitor spinal cord stimulator surgical patients to ensure they don't develop an adverse reaction or infection after surgery. These problems occur most frequently when medical and nursing staff fail to closely monitor post-operative spinal cord stimulator patients. The surgeon and nursing staff have a duty to monitor, recognize and react to any post-surgical complications.
Failing to Managing Patient's Condition Post-Operation
Before spinal cord stimulator surgery, your surgeon and anesthesiologist should provide you with information such as whether you are allowed to eat or drink and any substances you should avoid. When you are released home after surgery, your doctor should also provide you with important information, such as how to clean and care for your wounds, the medications you should take, and how much activity you can do without injuring yourself.

How Can Spinal Cord Stimulator Implant Cause Paralysis?
If a spinal cord stimulator is misplaced during surgery, a patient's spinal cord can be punctured or compressed by the stimulator's electrodes implanted in the epidural space. Both spinal cord punctures and pressure can cause paraplegia and quadriplegia. Sometimes the paralysis can be lessened or avoided if the surgeon and surgical team quickly recognizes the spinal cord compression and surgically “decompresses it” before permanent spinal cord damage is suffered.
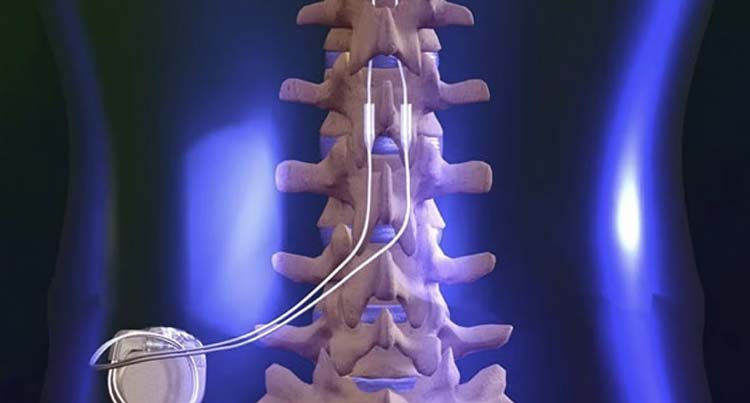
There are numerous scenarios where the insertion of a spinal cord stimulator may cause the patient to suffer from irreversible paralysis. For instance, if a surgeon is operating on a patient who has previously had a spinal cord stimulator placed, the surgeon may push down too hard against scar tissue from the previous stimulator while trying to insert the new paddle stimulator and cause the stimulator to deviate downward into the spinal cord, contusing it, and resulting in paralysis. The “standard of care” requires that the surgeon exercise extra care during this scenario to avoid injuring the spinal cord.
After surgery to implant or remove a spinal cord stimulator, critical issues like spinal bleeding or infection may develop and go undiagnosed if proper monitoring is not conducted. These conditions may arise in a few hours or days after the procedure. When medical personnel fail to properly recognize and diagnose the cause of the bleeding or infection after surgery, it can cause unnecessary harm due to medical malpractice.
Protecting the spinal cord at all costs is the cardinal rule for any surgeon and surgical nursing team performing surgery to implant or remove a spinal cord stimulator. The epidural, or area within which the stimulator is placed, is no more than a few millimeters wide. The spinal cord lies immediately below this space and can be easily injured if a piece of surgical equipment or paddle comes down on it with any force. In many cases where spinal cord stimulator injuries have led to paralysis, the surgeon placing the stimulator paddle encountered some resistance but inserted the paddle anyway.
According to The Wall Street Journal's analysis of adverse event reports submitted to the FDA, hundreds of patients who have had a spinal cord stimulator inserted in their back experienced partial or permanent paralysis due to complications many of which were caused by spinal cord stimulator medical malpractice.
Our client was paralyzed after spinal cord stimulator implant surgery. Our Medical Malpractice Law Firm obtained a $1,500,000 settlement or our client!.
Common Device Complications of a Spinal Cord Stimulator?
The most common hardware-related complications are lead failure and migration. Such problems are reported to be even more common than biological complications. Lead fractures are estimated to occur in 5-9% of devices, while spinal cord stimulator lead migration affects approximately 20-26% of patients.
Additional examples of device-related complications include:
- Injuries from lead breakage or migration
- Over or under-stimulation
- Intermittent stimulation
- Hardware malfunction
- Loose connections
- Battery failure
- Failure to communicate with the generator
One of the major complications that occur with spinal cord stimulators is infection, with incidences occurring in 3-10% of patients. Infection is a common cause of spinal cord removal and failure of therapy.
Common biological complications include:
- Risk of Infection: This risk is one of the most common, and if you get an infection around the implanted hardware, surgery may be required to treat the infection. In some cases, you may also need to have surgery to remove the generator and lead entirely.
- Pain Over the Implant Site: There may be pain over the implant site for approximately two weeks post-surgery, as the site may need time to adjust to the implant. Applying a cold compress over the incision site can reduce swelling, and pain medication can help alleviate symptoms.
- Epidural Hemorrhage: While epidural hemorrhages are rare following spinal cord stimulator implantation, the consequences of accessing the epidural space for anesthesia or interventional pain procedures can be potentially devastating. Some red flags for a hemorrhage that should raise suspicion in the post-operative period are weakness, numbness, and back and leg pain. It is critical that any problems that arise be identified and treated promptly. Treatment for an epidural hemorrhage typically involves surgery.
- Seroma: Occurs when fluid builds up around the device site and causes an infection. Sometimes, the body will reabsorb a seroma and resolve the issue without treatment. However, if the affected area presents with signs of infection, a doctor may perform a procedure known as aspiration, where the seroma is drained using a needle and a syringe.
- Paralysis: Patients who undergo spinal cord stimulator implantation are at risk of experiencing partial or permanent paralysis after having the device implanted. The treatment plan a doctor may prescribe will depend on the underlying causes and symptoms of paralysis. Treatment options may include taking medication for muscle spasms, stiffness, pain, and physical or occupational therapy.
- Dural Puncture: If a needle or electrode pierces the dura mater that surrounds the spinal cord, cerebrospinal fluid may leak out and cause severe headaches. Treatment options range from best rest as needed to epidural blood patches that may provide permanent symptomatic relief for a patient experiencing headaches.
- Neurological Damage: Direct trauma to the nervous system from a needle, lead, or paddle can result in a neurological injury and lead to paralysis. Treatment may involve taking medications and physical or occupational therapy.
- Cerebrospinal Fluid Leakage (CSF): Spinal fluid that leaks around the implant site may be caused by trauma such as a head injury or a medical error during brain or spinal surgery. The most common symptoms of a cerebrospinal fluid leakage are severe headaches or pain between the shoulder blades. Due to the increased risk of meningitis associated with CSF, you should visit your doctor if you're experiencing symptoms of CSF. Treatment options typically include an epidural blood patch or epidural patching with fibrin glue.
- Allergic Reaction to the Device: A patient with a history of significant allergic disease may develop an allergic reaction to the components of a spinal cord stimulator device. If conservative therapies fail to provide relief, removal of the device may provide definitive treatment of any associated symptoms.
Spinal cord stimulators are susceptible to a variety of issues; however, the most common complications are typically the result of device-related issues. A study conducted by the Therapeutic Good Administration of Australia reports that between July 2021 and January 2019, 520 patients suffered severe injuries linked to spinal cord stimulators.
According to an analysis of complications published by a University of Sydney researcher, 79% of these reported injuries were severe, and 13% were life-threatening. To reduce the risk of long-term injury, providers must be aware of the possible biological risks, select patients for trials based on appropriate criteria, and diagnose and treat developing complications early.
Proving Liability in a Spinal Cord Stimulator Negligent Implantation Case
A surgeon who implants a spinal cord stimulator into a patient's body has a responsibility to ensure that the spinal cord stimulation procedure is appropriately planned for, performed correctly and provide the patient with appropriate aftercare instructions.

A neuromonitoring company and neurologist has a responsibility to interpret the monitoring data and inform the surgeon of any spinal cord compression or injury. jIf you were injured by a spinal cord stimulator, you could be eligible to recover compensation for damages you've suffered by pursuing a medical malpractice lawsuit against a doctor or hospital responsible for the negligent implantation of a spinal cord stimulator.
To be eligible to pursue a claim for medical malpractice, you must be able to prove that a professional duty of care was owed to you. Whenever a doctor-patient relationship exists, the doctor has a duty to treat the patient with the utmost care and consideration. If a doctor negligently breached their duty of care by acting recklessly or carelessly during a medical procedure and made a mistake that severely harmed the patient, they could be held liable. We will also investigate any potential claims against a neuro-monitoring company, neurologist and even, where applicable, the manufacturer of the spinal cord stimulator device.
Medical malpractice lawsuits can quickly become very complex, and experts are often needed to testify as to whether the healthcare provider's actions or inaction fell below the appropriate standard of care. If you want to learn more about protecting your right to file a medical malpractice lawsuit, our lawyers are prepared to guide you every step of the way. At Miller Weisbrod Olesky, we offer free consultations where we will review the details of your case and advise you on the best course of action moving forward.
All spinal cord stimulator malpractice cases are handled by Miller Weisbrod Olesky on a contingency fee basis. This means you pay no fees or expenses unless we are successful in your case. We have a proven track record of success in spinal cord stimulator medical negligence cases and the expertise to find answers and hold negligent parties responsible for your injuries.
What Compensation Is Available in a Medical Malpractice Case?
Through a medical malpractice claim or lawsuit, you or a member of your family who suffered an injury after a spinal cord stimulator operation could be eligible to receive monetary compensation. If you decide to pursue a claim against the doctor or hospital responsible for your injury, you could recover compensation for the following damages and losses related to your spinal cord stimulator injury.
- Medical expenses (including past and future costs)
- Lost wages (including past and projected lost earnings)
- Mental, physical, or occupational therapy
- Assistive services (nursing care) and equipment
- Physical Impairment and Disability
- Emotional pain and suffering
- Loss of companionship
- Loss of consortium
- Loss of enjoyment of life
- Punitive damages
- Wrongful death
The details surrounding your claim will determine the specific type and amount of compensation you could be eligible to recover. For instance, the potential spinal cord stimulator settlement value of your case will depend on a wide range of factors, including the severity of your injury, the extent of the other party's liability, the impact the injuries have had on your life, and the amount of damages you've incurred. Each medical malpractice case is unique, and it's essential to consult an experienced spinal cord stimulator medical malpractice attorney who can help you gather the necessary evidence to support your claim and calculate the full extent of your losses.
Statute of Limitations for Medical Malpractice in Texas
The statute of limitations for medical malpractice cases is generally two years. This means that an individual must file a medical malpractice suit against someone no later than two years after the negligent act, omission, or practice occurred. However, it is essential to keep in mind that statutes of limitations vary based on the case being filed and the state where it's being filed.

Additionally, there are a few exceptions to the statute of limitations for malpractice cases. For instance, in some cases, the clock on the deadline may not start on the date of the incident, but on the date the injury was discovered. Consulting with a qualified medical malpractice attorney is the best way to ensure any deadlines that might apply to your claim are met on time.
Speak With a Spinal Cord Stimulator Medical Malpractice Lawyer Today
Miller Weisbrod Olesky is a national medical malpractice law firm based in Dallas that has successfully handled spinal cord stimulator cases in several states. We work with internationally recognized medical experts on spinal cord stimulator cases including neurosurgeons, neurologists, neuro-monitoring professionals, neuro-radiologists and physical medicine and rehabilitation physicians.

At Miller Weisbrod Olesky, every medical malpractice case we file is put through our intake and screening process and reviewed in detail by in-house nursing professionals and multiple outside experts.
Our strong history of proven results and large recoveries for victims of medical malpractice across Texas and nationwide is what sets our team apart from the competition. Let our compassionate and experienced attorneys fight to protect your rights and help you recover the compensation you deserve so you can move forward and focus on healing. Contact us toll-free at (888) 987-0005 or via our convenient online form to set up your free case evaluation.
Miller Weisbrod Olesky
Miller Weisbrod Olesky's Personal Injury Lawyers help individuals and families nationwide pursue real financial recovery after another's negligence causes an accident or medical mistake that results in serious personal injury or wrongful death.
Call our offices today at (888) 987-0005 for experienced assistance in a free consultation.
Useful Links
Testimonials
- Ms. Rosen: Mr. Weisbrod did a wonderful job on my son's case. I only wished that I had come to him sooner.
- A. Springs: Mr. Weisbrod was a delight to work with. His knowledge and memory was exceptional. He treated my case with sensitivity and care and I was always confident and never doubtful that he would win my case.
- D. Garcia: Whether you're in Dallas, Houston, or Austin, across Texas or around the nation, putting Miller Weisbrod on your legal team will push parties to settle.
Locations
★ Dallas
11551 Forest Central Drive, Suite 300
Dallas, TX 75243
★ Houston
12929 Gulf Freeway, Ste 111
Houston, TX 77034




